SWOG Cancer Research Network Clinical Trials Partnerships (SWOG CTP) is collaborating with AstraZeneca and Daiichi Sankyo as the scientific partners on their TROPION-Breast03 clinical trial.
The study is open at sites across the United States, including numerous SWOG member sites, and in more than a dozen other countries.
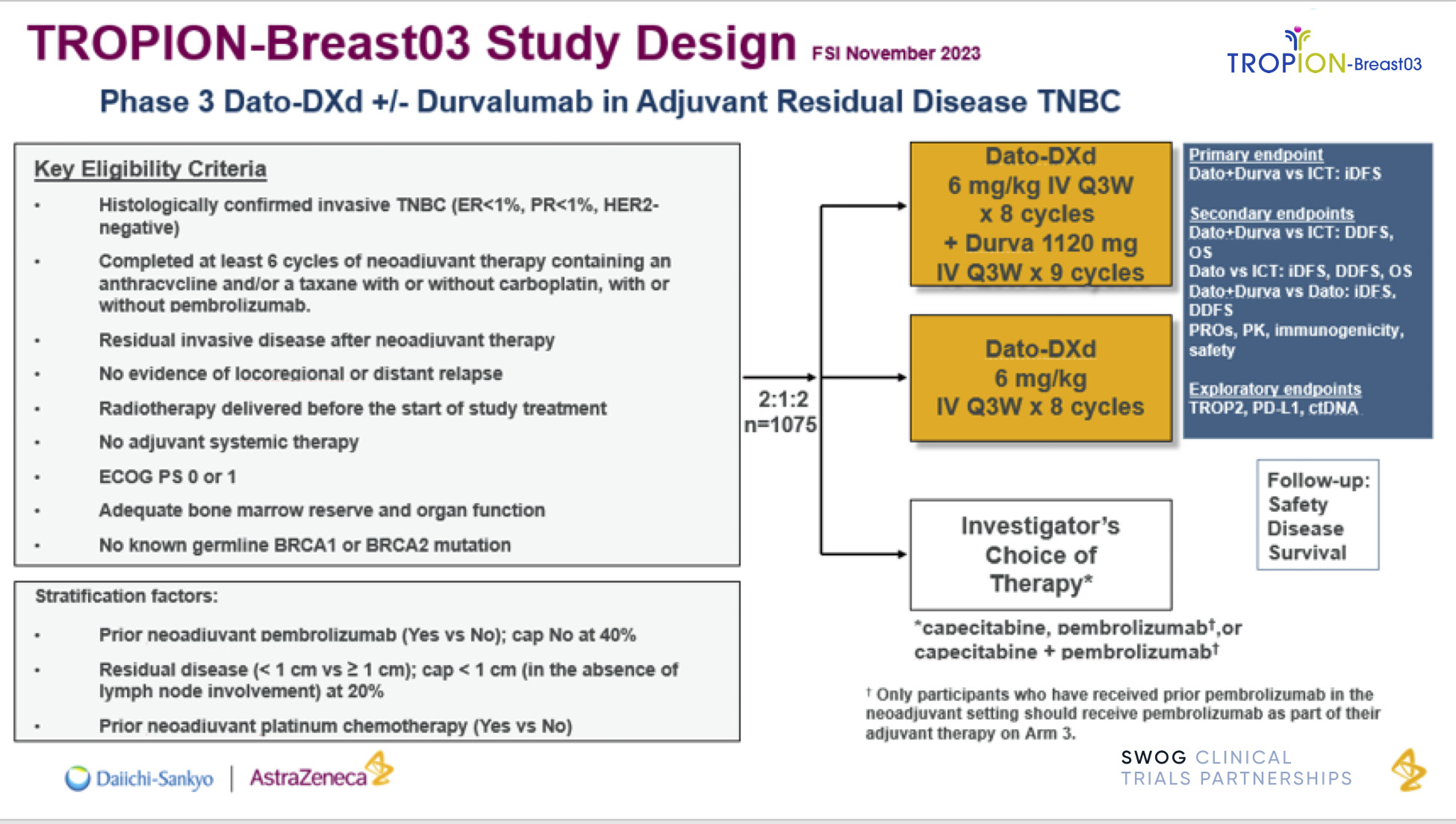
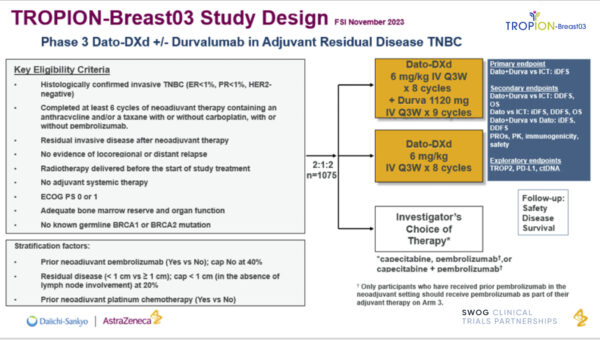
TROPION-Breast03 is a phase III, randomized, open-label, three-arm study assessing the efficacy and safety of datopotamab deruxtecan (Dato-DXd) with or without durvalumab compared with investigator’s choice of therapy (capecitabine and/or pembrolizumab) in participants with stage I to III triple-negative breast cancer (TNBC) with residual invasive disease in the breast and/or axillary lymph nodes at surgical resection following neoadjuvant systemic therapy.
The trial’s primary objective is to evaluate whether Dato-DXd in combination with durvalumab results in longer invasive disease-free survival (iDFS) than treatment with investigator’s choice of capecitabine and/or pembrolizumab.
The study chair is Aditya Bardia, MD, MPH, of Massachusetts General Hospital.
Further details are available on the ClinicalTrials.gov website (NCT05629585).
Also visit the trial website, available in English or Spanish.